 Thanks to the continuous and in depth Medical and Scientific Research, Medicap®4.0 Hair Implant our modern baldness solution for male and female alopecia problem or as hair thinning remedy, achieved the highly reliable results as reported in literature and as presented at many International conferences of Hair Restoration, Aesthetic Medicine, Cosmetic and Plastic surgery, Dermatologic Surgery, Anti Aging congress.
Thanks to the continuous and in depth Medical and Scientific Research, Medicap®4.0 Hair Implant our modern baldness solution for male and female alopecia problem or as hair thinning remedy, achieved the highly reliable results as reported in literature and as presented at many International conferences of Hair Restoration, Aesthetic Medicine, Cosmetic and Plastic surgery, Dermatologic Surgery, Anti Aging congress.
Clinical-histological studies at 2, 3 and 5 years confirm that THE ORIGINAL Medicap ® Biocompatible Artificial Hair achieves high quality and safety standards, such as negligible discomfort and inflammation levels, a high biocompatibility and resistance to chemical, physical and mechanical stress.
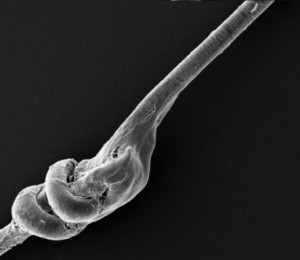
Further safety for the patient is ensured by the special Medicap® 4.0 Reversible knot that in case of need allows complete fibre removing without residues.
We are providing below some opinion expressed by some Authors of Clinical and Histological studies about the implantation of Medicap Biocompatible Artificial Hair, as worthwhile remedy for male and female alopecia and as hair thinning solution, reported in the referenced Medical Literature:
CLINICAL STUDIES
“….Omissis …..we believe that hair implant will be more widespread in the future enabling to restore alopecia and scarred areas of the scalp with satisfactorily cosmetic and psychological results”. Evaluation of polyamide synthetic hair. A long-term clinical study, Palmieri B., Griselli G., D’ugo A., Palmieri G., Salti G., Panminerva Med. 2000 March; 42(1):49-53
“….Omissis ….. It is a suitable methodology for surgeons, dermatologists and all those physicians who prefer a less traumatic and easier technique to satisfy those patients looking for an immediate aesthetic result….omissis ….. “. Implantation of Biocompatible Fibers for the Temporary Correction of Scalp Scars and Androgenetic Alopecia, Morselli M., Palmieri B., Santiago M., International Journal of Cosmetic Surgery and Aesthetic Dermatology 2003; 5(2):175-178
“….Omissis …..These new Polyamide Fiber Implants are well tolerated by the majority of the patients and the Authors agree that this is a possible adjunctive treatment for scalp scars and a viable alternative to nonsurgical solutions (toupees, hairpieces, wigs and similar)”. Artificial Hair Fiber Restoration in the Treatment of Scalp Scars – MA Santiago, R.Perez-Rangel,A. D’Ugo, G.Griselli, G.Igitian, I.Garcia Martin, G.B.Nesheim, Usama Saad Eddin,G.smith, G.W.Brady and C.Chacker. Dermatologic Surgery 2007; 33:35-44)
“….Omissis …..Artificial co-Polyamide Fiber Implantation is a safe, effective, easy and acceptable Hair Restoration Method in Androgenetic alopecia, especially in donor depleted cases”. AGRAWAL M., Modern Artificial Hair Implantation: a pilot study of 10 patients, Journal of Cosmetic Dermatology, 7(4): 315-323, July 3,2008)
HISTOLOGICICAL STUDIES
“…Omissis. Our study showed the same modifications in patients without inflammatory complications as in patients with inflammatory complications, except for the presence of inflammatory infiltrates. In our cases, the density of the fibers was lower than the one found in patients with cutaneous complications, in particular fibers were surrounded by closely adhering sleeves of keratin at the level of the pseudo-infundibula.
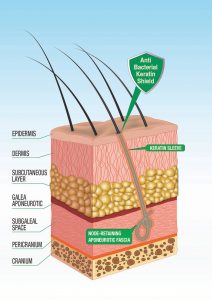 This aspect was absent in cases with inflammatory complications. In conclusion, if hairs are implanted at low density and the physical and chemical characteristics of the fiber allow the adherence of a sleeve of keratin inside the pseudo-infundibulum, it is possible that no clinically evident inflammatory complications will appear”. Fanti P.A., Pistorale T., D’urso C., Misciali C., Tosti A., Histological study on 5 cases of patients who underwent artificial hair implantation Without Complications, XXXII Annual Meeting, American Society of Dermatopathology, New Orleans, USA, February 01-03, 1995
This aspect was absent in cases with inflammatory complications. In conclusion, if hairs are implanted at low density and the physical and chemical characteristics of the fiber allow the adherence of a sleeve of keratin inside the pseudo-infundibulum, it is possible that no clinically evident inflammatory complications will appear”. Fanti P.A., Pistorale T., D’urso C., Misciali C., Tosti A., Histological study on 5 cases of patients who underwent artificial hair implantation Without Complications, XXXII Annual Meeting, American Society of Dermatopathology, New Orleans, USA, February 01-03, 1995
“….Omissis …..Implantation of Artificial Hair (Biofibre CE 0373/TGA marked) can be considered as the only alternative to treat Hair Loss for Women having severe rarefaction. The modest entity and nature of the phlogistic reaction demonstrates the high degree of biocompatibility of the fibers under examination. Rejection rate observed after pre-implant tests is acceptable (2%) and was completely solved by integrally extraction of the fibers” Santiago M., Histological study of the scalp implanted with polyamide Artificial fibers (Biofibre ® CE 0373/TGA marked). Analyses after 5 years, International Society of Hair Restoration Surgery, 10th Annual Meeting, Chicago, USA, October 9-13, 2002
For more information about the Products and Services by Medicap®





